In the vapour compression cycle shown in the figure, the evaporating and condensing temperatures are $260 \: K$ and $310 \: K$, respectively. The compressor takes in liquid-vapour mixture (state $1$) and isentropically compresses it to a dry saturated vapour condition (state $2$). The specific heat of the liquid refrigerant is $4.8 \: kJ/kg-K$ and may be treated as constant. The enthalpy of evaporation for the refrigerant at $310 \: K$ is $1054 \: kJ/kg$.
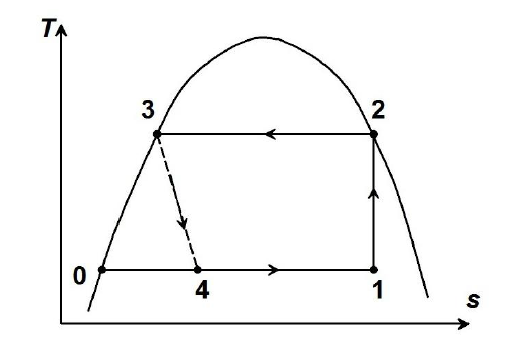
The difference between the enthalpies at state points $1$ and $0$ (in $kJ/kg$) is ____________