A closed vessel contains pure water, in thermal equilibrium with its vapour at $25^\circ C$(Stage$\#1$), as shown.
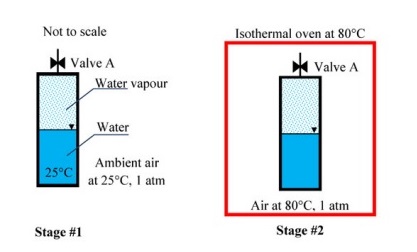
The vessel in this stage is then kept aside an isothermal oven which is having an atmosphere of hot air maintained at $80^\circ C$ The vessel exchanges heat with the oven atmosphere and attains a new thermal equilibrium (stage $\#2$). If the Valve $A$ is now opened inside the oven, what will happen immediately after opening the valve?
- Water vapor inside the vessel will come out of the Valve $A$
- Hot air will go inside the vessel through Valve $A$
- Nothing will happen – the vessel will continue to remain in equilibrium
- All the vapor inside the vessel will immediately condense